Obesity affects one in four adults in the UK, and this can lead to an increased risk of health conditions like type 2 diabetes, fatty liver or even certain cancers.
However, while some people’s risk of these conditions increases when they put on weight, we also know that some people can put on large amounts of weight and stay relatively healthy.
The level of risk varies for different people — and we don’t know why.
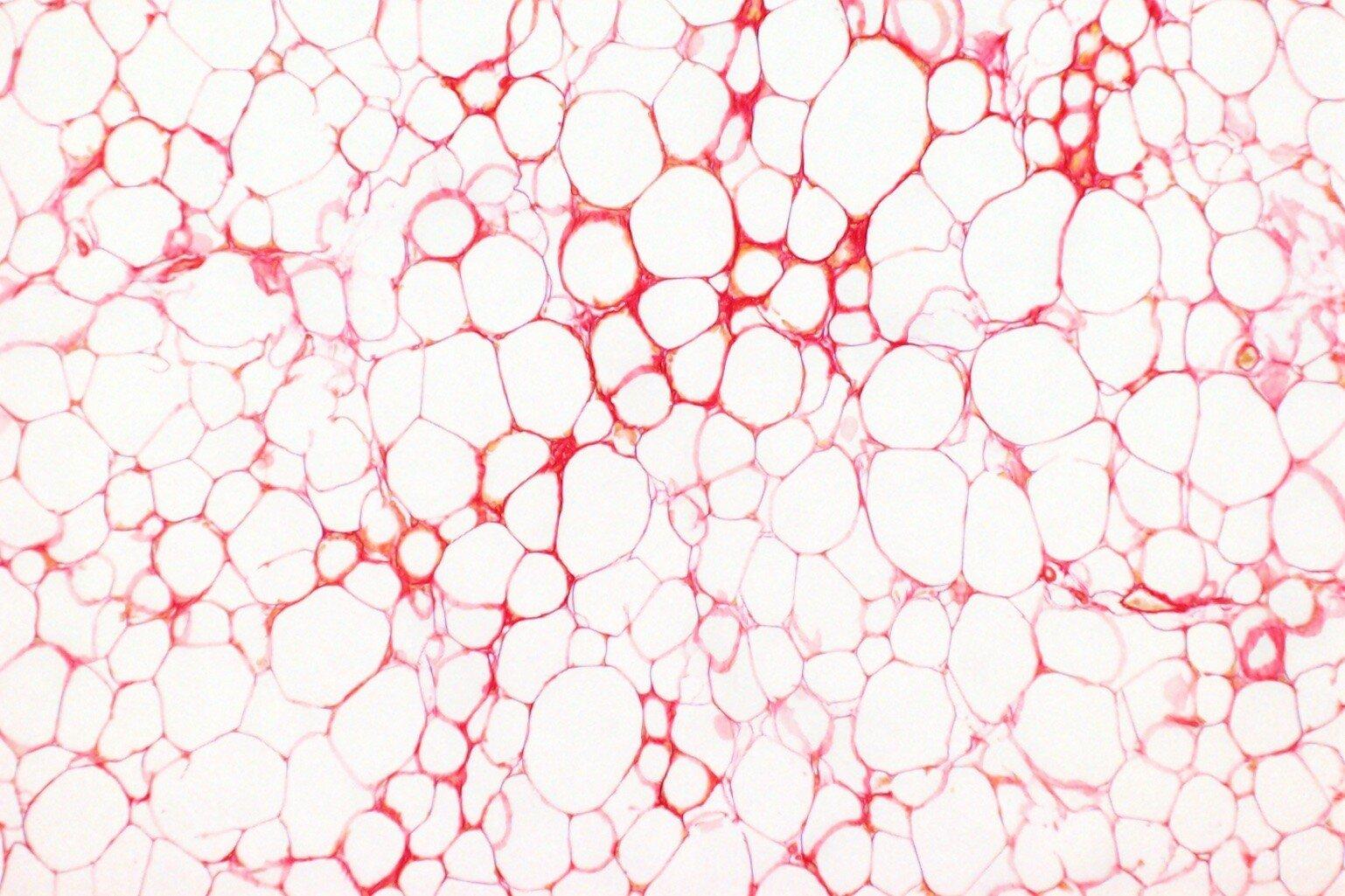
What happens to fat storage cells in obesity?
There's a lot of evidence that suggests our fat storage cells play an important role in determining why some people are more likely to develop health problems related to obesity.
One theory is that as obesity develops, our fat storage cells start to fail, which means our body doesn't store fat as safely as it should.
Normally, our fat tissue absorbs and releases fat as and when we need the energy or when we have an energy surplus. However, as obesity develops, that function can stop working as well. That's when people might accumulate fat elsewhere in their body, like in organs such as the liver, increasing the risk of organ dysfunction and disease.
But we really need to understand why that happens. What are the triggers for causing our fat storage cells to fail? And why does it only happen to some people?
That’s where the body clock might come in.
Uncovering the secrets of the body clock
We've known for some time that there is a link between metabolic disorders (like type 2 diabetes or obesity) and the disruption of our body clock. For example, research has shown that people who do rotating shift work are more likely to develop these conditions.
The body clock is a timekeeping mechanism that we have in almost every cell of the body. It's commonly associated with sleep cycles, but it also plays a critical role in regulating important metabolic processes in the body, including how fat cells function.
So, my research is trying to understand more about the behaviour of our fat storage cells, how their function is regulated by the body clock and how that might then influence our risk of obesity-related diseases.
More specifically, I’m investigating a protein within the body clock called Rev-erb-alpha.
Pressing play on the video above will set a third-party cookie. Please read our cookie policy for more information.
All the cells in our body have a timekeeping mechanism called the molecular clock – or the body clock. We know it’s important, but we don’t really understand how it impacts our health. Dr Louise Hunter is hoping to change that. She’s researching if our body clocks affect the way that our cells store fat, and it could fundamentally improve our understanding of health and disease.
Obesity is a significant public health problem. It affects one in three adults in the UK. We've known for some time that there is a link between the body clock and disruption of that body clock and risk of metabolic disorders. But what I'm hoping that my research will contribute to is a deeper understanding of the mechanisms by which the proteins that make up the molecular clock can regulate important metabolic processes in tissues such as fat tissue, and the liver for example.
My name's Louise Hunter, I'm a clinician, an endocrinologist, and I'm also a scientist here at the University of Manchester. My research is trying to understand more about the behaviour of our fat storage cells, our adipocytes, and how their biology and their response to the challenge of obesity is regulated by one of our body clock proteins.
I do experiments where I try and work out what that protein is doing in terms of how it's changing expression of certain genes, where it's binding to the DNA and how it's influencing the behaviour of the rest of the cell. Further on in my project, I'm planning to take some of my work into working with human volunteers and working with human tissue samples.
When people have obesity, it can put them at increased risk of certain conditions such as type 2 diabetes, fatty liver, it can even increase risk of certain cancers, and that's one of the reasons why obesity is such a problem.
It might not be the obesity itself, but it's the risk of other diseases that it brings with it. So, trying to understand how that risk comes about is really important. When we're trying to help people with obesity, it would be really nice to be able to do that in a way that's really tailored to them. So, understanding what their risks are.
The other direction in which I think this field could expand is in that deeper understanding of the body clock.
The molecular clock is a timekeeping mechanism present in all the cells of our body. We know it's really important, but how does that translate to human health? Can we get our knowledge of the body clock to a point where we can really effectively exploit that knowledge to improve human health?
As a clinician, I want my research to be relevant to people and their health conditions, but we do really need to have a fundamental understanding of health and disease processes and that needs discovery research.
Using molecular biology processes in the lab, I conduct experiments to uncover what this protein is doing to change the expression of certain genes and how it influences the behaviour of the fat cell and the rest of the fat tissue. We also work with animal models, and further on in my project, I'm planning to work with human volunteers and human tissue samples.
Discovery research to improve human health
As a clinician, I want my research to be relevant to people and their health conditions. I’m fascinated by digging into the nitty gritty of how our bodies work. At every level that you go to, there's always a further level of detail to try and understand.
This type of research is discovery research – it’s about trying to understand the fundamental processes that underlie what happens in health and disease. The better understanding we have of the basic processes, the better we can apply that knowledge to improve human health.
That's why I was drawn to Wellcome’s Early Career Award, because of the possibility and encouragement to pursue discovery research.
Ultimately, I hope that my research will improve our understanding of how to help people who develop obesity-related diseases. I want to get to a stage where we can personalise treatment tailored to their unique risks of developing certain conditions.