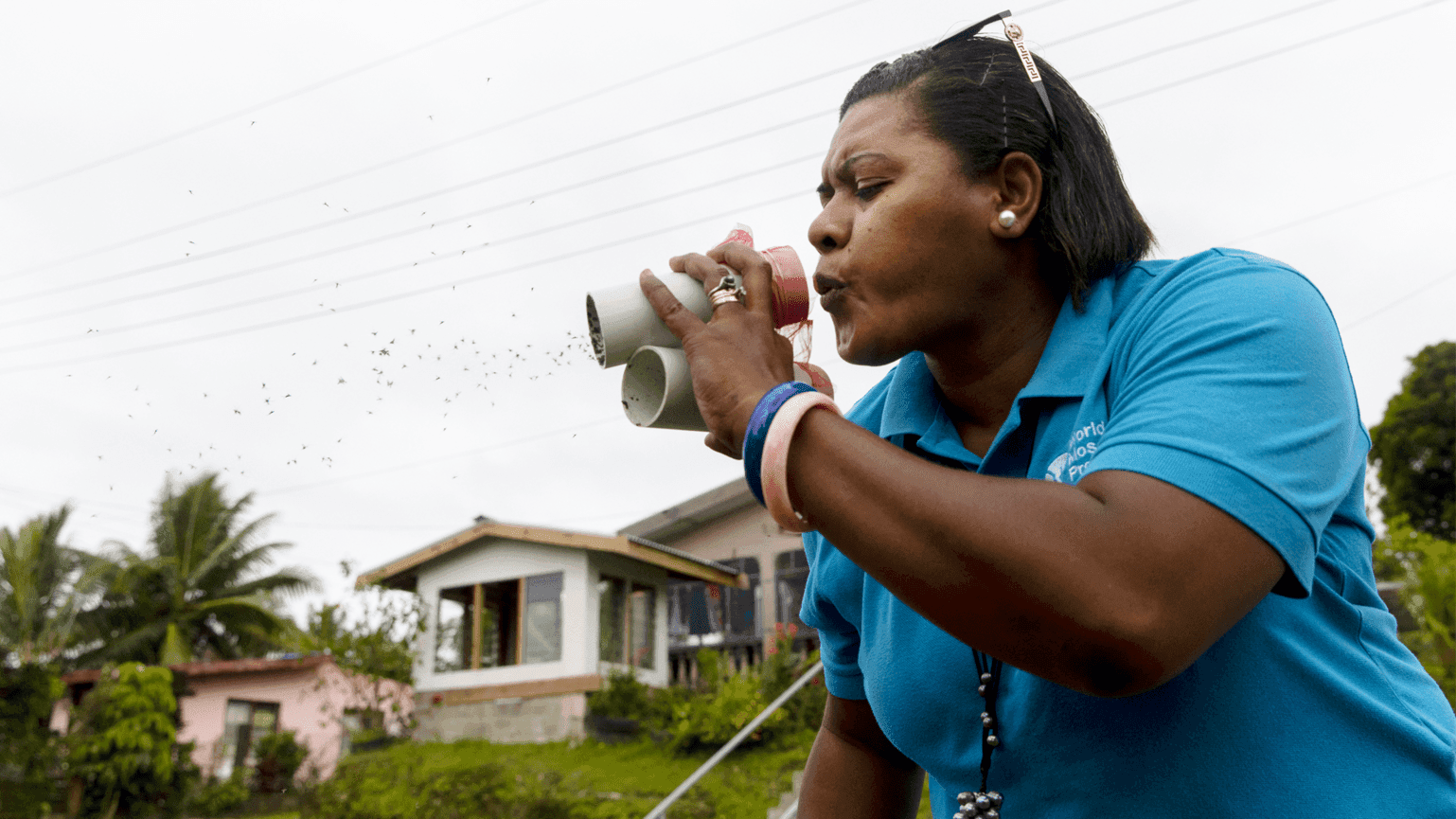We fund research that aims to better understand the reservoirs of disease and the factors that drive disease escalation – such as global connectivity, climate change, and the overuse of antibiotics.
We also focus on how to best map and measure these sources and drivers through improved surveillance.
We’re interested in funding projects that enable us to transform the field of Research and Development to ensure the creation of accessible, affordable, and available products.
We also award grants to studies that create the evidence we need to persuade key decision makers, such as local and national governments, to make science-informed choices when devising policy around human disease.
Additionally, we fund discovery research in a broad range of disciplines, including infectious disease.
We also invite organisations to apply for contract opportunities that support our mission.